UndefinedNameError: reference to undefined name 'HideElements' (click for details)Callstack:
at (Workbench/LarsenLab/Research/PhotoBiology/Photoreceptors), /content/body/pre, line 1, column 1
Photoactive Yellow Protein (PYP) is a small, 125 residue, water-soluble protein and a member of the xanthopsin photoreceptor family. PYP can be isolated from the purple phototropic eubacterium Halorhodospira halophila, and is presumed responsible for the initial steps of the homeostatic pathway that results in the negative phototaxis of the bacterium to blue light. In the past decade, PYP has become a model system for studying the photo-initiation and ensuing dynamics of photoreceptor proteins. We are particularly interested in three aspects of the PYP photocycle:
- Primary photochemistry
- Functional protein dynamics and unfolding
- Signaling state formation, particularly within the context of the common structure of the PAS-domain family.
PYP Photocycle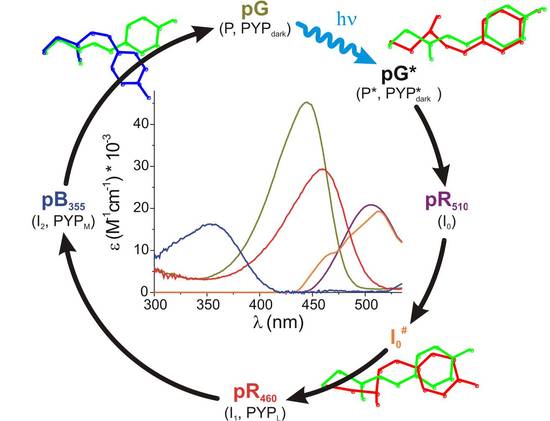
Functional activation of PYP can be measured in vitro through analysis of its photocycle with a wide range of biophysical techniques. Following the absorption of a photon, the embedded p-coumaric acid (pCA) chromophore starts to twist in the excited-state surface and undergoes a full trans-cis isomerization before internally converting to the ground-state via a conical intersection with a ~30% reaction yield. A number of red-shifted intermediates with the chromophore in cis configuration are formed on picosecond-microsecond timescales. On the millisecond timescale, the chromophore is transiently and reversibly protonated by the neighboring carboxyl group of E46. As a result, the protein partially unfolds (and subsequently refolds), while simultaneously shifting its color to the blue (350 nm). These events are thought to form the signaling state of PYP.


